Claims about fluoride and cancer
This will be a long one. But the interesting stuff comes at "Dr John Yiamouyiannis". Skip to there to see some interesting data.So, fluoride in tooth paste is apparently good against caries. But people I have been talking to, who refuse to use fluoridated tooth paste, claims that fluoride causes cancer. Again, I think these claims have no ground. I have asked them to show me epidemiological studies showing the relationship of fluoride and cancer (we have been using fluoride since 60's, so there should be a LOT of data).
The answers I keep receiving are as follows
- The big companies does not want you to know the truth
- My answer to this is: Well, yes I can agree to that to certain extent. Big companies does not want their product to have a bad reputation, but at the end, the truth will reach the consumers. See what happened with cigarettes, freons, carbon dioxide, eutrophication, acid rain etc.
- There are scientific studies showing the dangers of fluoride
- I do always ask for those papers. Here are some of those I have got:
The concentration of fluoride in our body is around 0,01-0,03 ppm (ca 0,01-0,03 mg/L), although it can be higher in our bones. In the studies referred above, they use > 50 mg / L fluoride (ca 50 ppm), e.g. much higher concentrations to what is likely to be found in the human body.
Dr John Yiamouyiannis
I got also a link to a paper written by Dr John Yiamouyiannis, as a proof of correlation between fluridation of water and cancer. http://www.fluorideresearch.org/103/files/FJ1977_v10_n3_p095-148.pdf
Apparently, this guy is the guru of correlation between cancer and fluoride in drinking water. This means I will deviate from the topic "fluoride in tooth-paste" to the topic "fluoride in drinking water"
Apparently, this guy is the guru of correlation between cancer and fluoride in drinking water. This means I will deviate from the topic "fluoride in tooth-paste" to the topic "fluoride in drinking water"
In this paper, they compared the mortality rate for cancer in the 10 largest fluoridated US cities to those of the 10 largest non-fluoridated US cities in the same time period. The idea was that other factors would be nullified as people in large cities have similar living habits. Thus, the differences in cancer deaths could be related to fluoride if any difference was found. Anyway, the authors found that there was a difference between the fluoridated and non-fluoridated cities, and they claimed that this difference was related to the fluoride in water that was added.
When I first read the paper I though "hmmm, interesting". But I did question the validity of their conclusions:
When I saw these numbers and took a quick glance at the tables, I wondered: "15/100000 and 35/100000, is that REALLY a significant increase that can ONLY be related to fluoride in drinking water?
I decided to go deeper. I started to examine the tables and saw that the correlation coefficients they were using to confirm their claims were quite low (see tables 4 and 5, there raw data is there)
I used a simple regression analysis to start with. I took the average values of death rates of fluoridated cities vs the average values of death rates of non-fluoridated cities. This is what I got:
Just looking at the averages, you could say there is a very weak correlation between cancer occurrence and fluoridation, but VERY weak (correlation coefficient is 0,26). Almost no correlation was found for the non-fluoridated cities (corr coeff 0,005).
So, I decided to go deeper into the data and go through it city by city. Lets start with the fluoridated cities.
OMG! The correlations are still positive, but none of them have a correlation coefficient above 0,2!!! In other words, there is no correlation to talk about! But this is not all. The biggest surprise in this group was the city of Milwauke. As you can se below, the correlation is NEGATIVE, and the correlation coefficient is above 0,2 (if that means something)!
So, does this means that non-fluoridation is as dangerous as fluoridation of water? NO! This means that this study has not found any evidence for why the death rates are increasing! That is, Yiamouyiannis study does show nothing!
Just for the sake of consistency, here are the results of the other non-fluoridated cities:
- They found an increase in cancer death rate by 15 out of 100 000 persons in the population aged 45-64 years. Also, an increase by 35 out of 100 000 persons in the population ager 65 or more (65 +)
- No significant increase was extracted from the data in the population 0-24 years and 25-44 years
When I saw these numbers and took a quick glance at the tables, I wondered: "15/100000 and 35/100000, is that REALLY a significant increase that can ONLY be related to fluoride in drinking water?
I decided to go deeper. I started to examine the tables and saw that the correlation coefficients they were using to confirm their claims were quite low (see tables 4 and 5, there raw data is there)
I used a simple regression analysis to start with. I took the average values of death rates of fluoridated cities vs the average values of death rates of non-fluoridated cities. This is what I got:
Just looking at the averages, you could say there is a very weak correlation between cancer occurrence and fluoridation, but VERY weak (correlation coefficient is 0,26). Almost no correlation was found for the non-fluoridated cities (corr coeff 0,005).
So, I decided to go deeper into the data and go through it city by city. Lets start with the fluoridated cities.
Fluoridated cities
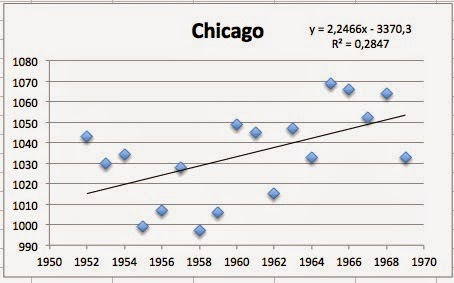
The 10 studied cities were Philladelphia, Baltimore, Claveland, Washington, Milwauke, St Louis, San Francisco, Pittsburg, Buffalo
From the data, only three cities showed a correlation coefficient above 0,2! All other cities had corr coeff below this number. And, lets be sincere. Does a corr coeff below 0,4 really mean anything? With this said, let us take a closer look.
So Claveland, Baltimore and chicago all had a positive correlation, and correlations coefficients above 0,25. So far, Yiamouyiannis claims might seem to be valid. But what about the rest of the cities?
OMG! The correlations are still positive, but none of them have a correlation coefficient above 0,2!!! In other words, there is no correlation to talk about! But this is not all. The biggest surprise in this group was the city of Milwauke. As you can se below, the correlation is NEGATIVE, and the correlation coefficient is above 0,2 (if that means something)!
But this is not all. More surprises are to come in the non-fluoridated cities
Non-fluoridated cities
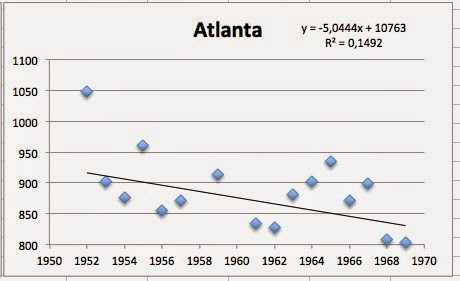
The non-fluoridated cities were Los Angeles, Boston, New Orleans, Seattle, Cincinnati, Atlanta, Kansas City, Columbia, Newark and Portland
I did the same type of correlation analysis and got a BIG surprise. Three of the non-fluoridated cities had a positive correlation between dead-rates and non-fluoridation of water (if you want to translate the results that way):
So, does this means that non-fluoridation is as dangerous as fluoridation of water? NO! This means that this study has not found any evidence for why the death rates are increasing! That is, Yiamouyiannis study does show nothing!
Just for the sake of consistency, here are the results of the other non-fluoridated cities:
In conclusion
To conclude. There is no evidence that fluoridation of water causes cancer given by studies. There is a much better paper by a japanese team. They have found a very weak correlation between fluoride and some types of cancer.
But as they say themselves: "Finally, we must conclude that the consistency of the fluoride-associations for cancers and temporal relationship are not yet adequately confirmed because of its sociological conditions. We would like to ask for the cooperation of researchers throughout the world to further assess fluoride as a genetic cause of cancers from the standpoint of epidemiology and also in animal experiments. so as to strengthen the power of five criteria and stop the application of fluoride for prevention of teeth caries if this indeed presents as a risk factor for cancer"
In other words, they are unsure of their results and more data is needed.
Therefore, the small amounts of fluoride you get from tooth paste does not pose a treat for you or your children, if administrated as intended!
In other words, they are unsure of their results and more data is needed.
Therefore, the small amounts of fluoride you get from tooth paste does not pose a treat for you or your children, if administrated as intended!