Well, I would not but it did. It turns that a very very very small amount of this LDA actually can give you impurities (ca 0.28 area % in my case) due to oxidation of the base.
To give you a more specific example, if you have an ester, and you use LDA to pick a proton in another part of your target molecule, do not get puzzled if you get things like
 |
| Side reaction of esters and LDA |
Of course, most of the LDA will act as a base and the desired product will be the major product you will obtain. But when looking at impurities is important, it is easy to overlook at this one.
So, what is actually happening. According to Férézou and Chevalley (Tetrahedron 68 (2012) 5882-5889), LDA can be oxidized by interaction with aldehydes and ketones (they have used benzophenone and benzaldehyde, among others, as oxidazers). A plausible mechanism is presented below:
 |
| First, the oxidative agent coordinates with Lithium, reducing the alcohol and oxidizing LDA, you get an imine |
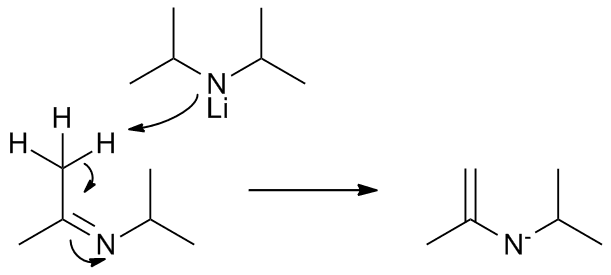 |
| Another LDA molecule acts as base, attacking one of the nitrogens next to the imine carbon. An enamine is created. |
 |
| Through resonance, the enamine carbon acts as nucleophile, attacking the electrophilic carbonyl carbon. This would yield the bi-product. This mechanism is not confirmed but is fully possible. |

